Amazing Micture Recipes & Inspiration
What is the nature of a blended composition? A comprehensive understanding of combined elements is crucial for analysis.
The term refers to a complex substance formed by combining two or more distinct elements. A mixture, unlike a compound, retains the individual properties of its components. For example, a salad dressing, composed of oil, vinegar, and herbs, is a mixture; the ingredients maintain their own identities while blended together. Similarly, sand mixed with water exemplifies a mixture, and the water and sand maintain their individual properties.
Understanding the characteristics of mixtures is foundational in numerous scientific disciplines. From chemistry and material science, to environmental science and food preparation, the study of mixtures provides vital knowledge. The properties of a mixture, such as its density, color, or texture, often depend on the proportions of the components. Analyzing these blends is essential for predicting and controlling outcomes in various settings. In food preparation, blending ingredients impacts taste, texture, and the overall sensory experience. Chemical interactions within the mixtures can influence their stability over time.
Moving forward, we will explore various types of mixtures, their applications, and their differences from compounds, focusing on their practical utility.
Mixture
Understanding the fundamental characteristics of mixtures is crucial for various scientific and practical applications. This includes comprehending their composition, properties, and behaviors.
- Composition
- Properties
- Separation
- Homogeneity
- Heterogeneity
- Applications
A mixture's composition details the constituent elements or substances. Properties, like density and color, arise from the combined characteristics of these components. Separation techniques are vital for isolating individual components within mixtures. Homogeneity refers to a uniform distribution of constituents throughout, exemplified by saltwater. Heterogeneity, conversely, denotes an uneven distribution, as in a salad. Mixtures find applications in various fields, from food preparation to industrial processes. For instance, alloysmixtures of metalsexhibit combined properties crucial in engineering.
1. Composition
Composition, in the context of mixtures, dictates the nature and properties of the resultant blend. The precise quantities and types of components forming a mixture fundamentally define its characteristics. A mixture of sand and water, for example, possesses distinct properties from a mixture of sugar and water, owing to the differing compositions. Understanding the quantitative and qualitative makeup of the constituents is paramount for anticipating the behavior and outcomes of a mixture. For instance, the concentration of solute in a saltwater solution directly impacts its density and conductivity.
The importance of composition in mixtures extends beyond basic observation. In industrial settings, precise control over mixture composition is critical for achieving desired product outcomes. Consider the creation of alloys. The precise proportions of metallic elements in an alloy significantly affect its strength, durability, and corrosion resistance. Similar principles apply in pharmaceutical formulations, where the precise composition of ingredients dictates the drug's efficacy and safety. In food science, carefully balanced mixtures of ingredients ensure the desired taste, texture, and nutritional content. Moreover, environmental applications, like water treatment, require the precise understanding and control of mixture composition to achieve desired purification results.
In summary, composition is the foundational aspect of mixtures. Accurate knowledge of the constituent elements, their ratios, and inherent properties is paramount for understanding and manipulating the properties and behaviors of mixtures. A thorough comprehension of composition is not only essential for theoretical understanding but also for practical applications in diverse fields. Miscalculations in composition can lead to undesirable outcomes, ranging from suboptimal product quality to safety hazards. Thus, careful consideration and precise control over mixture composition are crucial to achieving desired results and preventing unintended consequences.
2. Properties
The properties of a mixture are a direct consequence of the combined characteristics of its constituent elements. Understanding these properties is crucial for predicting and controlling the behavior of the mixture, whether in a laboratory setting or an industrial process. This understanding is essential for applications ranging from food preparation to materials science. The interplay of constituent properties shapes the overall profile of the mixture.
- Physical Properties
Physical properties, such as density, melting point, and boiling point, are often additive or affected by the proportions of components in a mixture. For instance, the density of a saltwater solution varies directly with the concentration of salt. Similarly, the boiling point of a mixture of two liquids might not fall exactly between the boiling points of the individual components but rather depend on their relative amounts. Such variations are key considerations in processes like desalination and solvent extraction.
- Chemical Properties
The chemical properties of a mixture, however, do not simply add together. While individual components might be chemically inert, their interaction within the mixture can sometimes yield surprising results. Reactions can proceed at different rates depending on the environment provided by the mixture. For instance, a mixture of hydrogen and oxygen, while individually stable, can react explosively when ignited. This is a fundamental difference between mixtures and compounds, where the chemical characteristics of the compound are significantly altered from its constituents.
- Mechanical Properties
The mechanical properties of a mixture are defined by its response to external forces. For example, the strength and stiffness of a composite material are determined by the combination of its components. The interplay between the constituents influences the overall mechanical properties, which are critical in engineering applications. The strength of concrete, for example, relies on the interplay of cement, aggregate, and water.
- Thermal Properties
Thermal properties, including heat capacity and thermal conductivity, are also influenced by the composition of a mixture. The overall thermal response of the mixture often reflects the properties of its components. The ability of a mixture to transfer heat can be quite different than that of its components. This effect is relevant in situations requiring good thermal insulation or heat exchange.
In conclusion, the properties of a mixture are not simply a summation of its constituents' individual characteristics. The interactions between components often give rise to novel and significant emergent properties. Understanding these multifaceted properties is essential for controlling and manipulating mixtures effectively across diverse fields.
3. Separation
Separation techniques are integral to the study and utilization of mixtures. The ability to isolate individual components from a mixture is fundamental to many scientific and industrial processes. This separation process allows for the identification, analysis, and eventual exploitation of the unique properties inherent in each constituent.
- Physical Separation Methods
Numerous physical methods exist for separating components of a mixture. Techniques such as decantation, filtration, distillation, and evaporation exploit differences in physical properties, like density, boiling point, or solubility, to isolate desired substances. Decantation, for example, separates a liquid from a solid by carefully pouring off the liquid; filtration utilizes porous materials to retain solids; distillation leverages differing boiling points to separate liquids; and evaporation concentrates a substance from a solution by heating.
- Chromatographic Techniques
Chromatography provides a more sophisticated approach to component separation. Various types of chromatography, such as thin-layer chromatography (TLC), gas chromatography (GC), and high-performance liquid chromatography (HPLC), separate substances based on their differential affinities for a stationary and mobile phase. This method is particularly useful in complex mixtures containing multiple components with similar physical properties, such as separating pigments from plant extracts or identifying different chemical compounds in a sample.
- Role in Analysis
Separation plays a critical role in analytical chemistry. Analyzing the composition and characteristics of components often requires their isolation from the mixture. This isolation allows for specific testing, identification, and quantification. For example, separating pollutants from water allows for precise measurement of concentration and potential harm.
- Industrial Applications
Separation techniques are ubiquitous in industrial settings. From the refining of petroleum to the production of pharmaceuticals, separation techniques are employed to isolate desired substances and purify raw materials. For example, the petroleum industry uses fractional distillation to separate crude oil into various usable components, such as gasoline and diesel fuel.
In summary, separation techniques are fundamental to the understanding and manipulation of mixtures. These methods, whether simple or advanced, allow for the isolation of individual components, unlocking their unique properties and applications in a multitude of sectors. The successful application of these techniques hinges on an accurate understanding of the physical and chemical differences between components within a mixture.
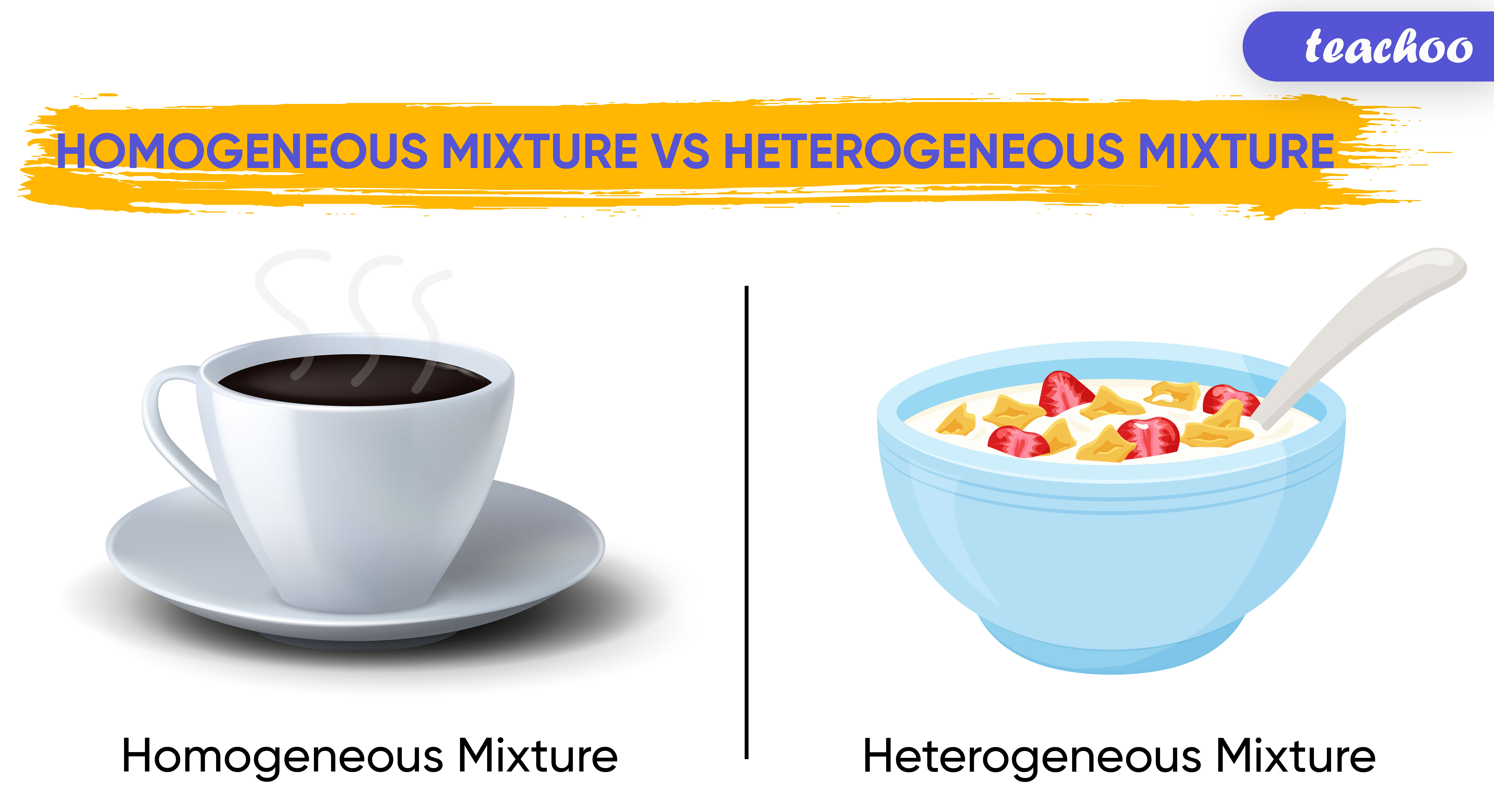
4. Homogeneity
Homogeneity, in the context of mixtures, describes the uniform distribution of components throughout the substance. A homogeneous mixture exhibits a consistent composition throughout, meaning the properties are the same at any point within the mixture. This uniformity is a defining characteristic distinguishing homogeneous mixtures from heterogeneous mixtures, in which components are not evenly distributed and visibly distinct phases exist. The concept of homogeneity is intrinsically linked to the understanding and manipulation of mixtures. Understanding homogeneity enables predictions about the behavior and properties of a substance.
The importance of homogeneity stems from its impact on the properties and behavior of the mixture. In a homogeneous mixture, properties like concentration, density, and color remain constant throughout the sample. This consistency is crucial in various applications. For example, the concentration of salt in a saltwater solution is uniform throughout, making it predictable and useful for specific applications. Similarly, the consistent composition of air, while complex, allows for reliable predictions regarding atmospheric pressure and temperature gradients. Homogeneous mixtures are frequently utilized in industrial processes, like chemical manufacturing and pharmaceutical production, where the consistency of the final product is vital.
In summary, homogeneity in a mixture reflects a uniform distribution of components. This uniformity directly influences the measurable properties of the mixture. Understanding homogeneity is fundamental to predicting and manipulating the behavior of mixtures, thus enabling successful applications in various scientific and industrial settings. Variations in homogeneity can lead to inconsistencies in properties, potentially impacting the outcome of processes relying on consistent compositions. Thus, achieving and maintaining homogeneity is crucial in many scientific and industrial applications.
5. Heterogeneity
Heterogeneity, a defining characteristic of certain mixtures, signifies the non-uniform distribution of components. Unlike homogeneous mixtures, where composition remains consistent throughout, heterogeneous mixtures exhibit distinct phases or regions with varying compositions. This inherent unevenness influences the observable properties and behavior of the mixture. The presence of distinct phases, often visually apparent, creates a complex system that differs fundamentally from the uniform nature of homogeneous counterparts.
Heterogeneity is not simply a matter of aesthetic appearance; it has practical implications. The separation of components in heterogeneous mixtures often necessitates distinct techniques, different from those applied to homogeneous blends. Consider a mixture of sand and water. The sand particles are dispersed throughout the water, forming a visible, non-uniform distribution, requiring filtration to isolate the sand from the water. This contrast in distribution directly dictates the choice of separation method. Similarly, in a rock sample, different minerals are often visibly segregated, leading to localized variations in mineral composition. The implications of this segregation influence analysis for geological studies, while the understanding of localized compositions is fundamental to understanding the origin and development of that geological formation. Likewise, in soil samples, the varying proportions of organic matter and mineral components across different zones influence nutrient availability and overall soil fertility.
In conclusion, heterogeneity within a mixture is a significant factor determining its characteristics and the methods necessary for analysis and application. The presence of distinct phases and non-uniform distribution necessitates tailored approaches for both separation and understanding. Recognizing heterogeneity is critical for accurate interpretation of complex systems, guiding the selection of appropriate techniques and enabling predictive analysis across various fields, from environmental science to materials engineering and beyond.
6. Applications
The practical applications of mixtures are extensive and diverse, encompassing numerous fields. The properties of a mixtureresulting from the combined characteristics of its constituentsunderpin numerous processes and products. A clear understanding of these applications is crucial to harnessing the potential of mixtures effectively.
- Food and Beverage Production
Mixtures are fundamental in food preparation and beverage production. From baking a cake (a complex mixture of flour, sugar, eggs, and other ingredients) to brewing coffee (a combination of water, coffee beans, and often other additives), mixtures determine the final product's texture, flavor, and nutritional value. Precise control over the composition of these mixtures is essential to ensure consistent quality and desired outcomes.
- Material Science and Engineering
The creation of alloys, composites, and other advanced materials relies heavily on the principles of mixtures. The mixing of different metals (as in steel), or combining polymers with fillers (as in reinforced plastics), creates materials with enhanced properties, such as increased strength, durability, or resistance to corrosion. Controlling the composition and mixing processes is critical in achieving the desired characteristics of these engineered materials.
- Environmental Science and Technology
Mixtures play a significant role in environmental science and technology. Water treatment involves separating impurities and contaminants from water, often relying on the distinct properties of different substances within the mixture. Understanding the composition and properties of air pollutants allows for targeted mitigation strategies and pollution control. The remediation of contaminated sites involves carefully designed mixtures to facilitate the removal of pollutants.
- Chemical Manufacturing and Pharmaceuticals
Chemical manufacturing and the development of pharmaceuticals frequently involve complex mixtures. The synthesis and formulation of specific chemical compounds often require carefully controlling the proportions of reactants in a reaction mixture. Precise mixtures are critical in the formulation of medications, ensuring accurate dosage and optimal efficacy. The stability and purity of these mixtures are essential to the drug's effectiveness and safety.
In summary, the ubiquitous nature of mixtures extends far beyond basic examples. From sustenance to complex technological advancements, understanding and manipulating the components and properties of mixtures is central to numerous industrial and scientific endeavors. The precise control and optimization of these mixtures determine the quality, performance, and effectiveness of countless products and processes.
Frequently Asked Questions About Mixtures
This section addresses common inquiries regarding mixtures, providing concise and informative answers to clarify key concepts and dispel potential misconceptions.
Question 1: What distinguishes a mixture from a compound?
A mixture comprises two or more substances combined physically, retaining their individual identities and properties. A compound, in contrast, results from a chemical reaction where elements combine in fixed proportions, forming a new substance with entirely different characteristics than its constituent elements. The key difference lies in the nature of the bonding and the resulting properties.
Question 2: How are the properties of a mixture influenced by its composition?
The properties of a mixture are a direct reflection of the components present and their proportions. Physical properties, such as density and melting point, often exhibit additive or proportional relationships relative to the components' individual properties. Chemical properties, however, are not simply additive and can be significantly altered due to interactions between components within the mixture.
Question 3: What techniques are employed to separate components of a mixture?
Various methods exist for separating mixture components. Techniques such as filtration, distillation, decantation, and evaporation leverage differences in physical properties, like solubility, boiling point, or density. Chromatography provides a more sophisticated approach, employing differential affinities between components and a stationary or mobile phase to achieve separation.
Question 4: Are all mixtures heterogeneous?
No, not all mixtures are heterogeneous. Homogeneous mixtures exhibit a uniform composition throughout, with components indistinguishably distributed. Saltwater, for example, is a homogeneous mixture, while a mixture of sand and water is heterogeneous due to the visible separation of phases.
Question 5: What are some practical applications of mixtures?
Mixtures have widespread applications in various fields. Food preparation, material science, environmental remediation, and pharmaceutical formulation all utilize mixtures. Understanding and controlling the properties of mixtures is crucial for achieving desired results in these and many other applications.
In summary, a thorough comprehension of mixtures, encompassing their composition, properties, and separation techniques, is vital for various scientific and practical endeavors. This understanding is crucial for various industries and scientific research.
Moving forward, we will explore specific types of mixtures and their detailed applications.
Conclusion
This exploration of mixtures highlights the fundamental importance of understanding their composition, properties, and separation techniques. Mixtures, encompassing a vast array of substances from simple solutions to complex alloys, exhibit emergent properties that are not simply the sum of their constituent parts. The uniform distribution of components in homogeneous mixtures contrasts sharply with the distinct phases observed in heterogeneous systems, influencing the selection of appropriate separation methods. The diverse applications of mixtures span numerous fields, from food preparation and material science to environmental remediation and pharmaceuticals. Properly understanding and manipulating mixtures is crucial to achieving desired results across these sectors.
The significance of mixture analysis extends beyond practical applications. The intricate interplay of components within mixtures provides valuable insights into the behavior of matter at a fundamental level. Further research into the nuanced interactions within various types of mixtures holds potential for developing novel materials and processes. A deeper understanding of these interactions promises to contribute to advancements in areas like sustainable energy, efficient resource utilization, and improved healthcare.
- Adorable Beagle Mix Puppies For Sale Find Your Perfect Friend
- Adorable Little Izzy Fun Photos Videos
Homogeneous Vs Heterogeneous Matter Worksheets
/GettyImages-594836477-58fe53963df78ca159068b1a.jpg)
Mixture Definition and Examples in Science

Pin by Женя Фрезер on To make Hot wheels wall art, Hot wheels wall